Massachusetts Training Center (MTC)
- HOME
- Massachusetts Training Center (MTC)
Massachusetts Training Center
The Massachusetts Training Center exists to provide our customers with timely accurate information and support on Asahi Kasei Bioprocess products along with the knowledge to integrate these products into a robust manufacturing process.We offer globally connected technical trainings in the use of virus filtration/microfiltration products and concept driven learning in biological processes through an innovative and top tier approach to customer experience.
We are a team of scientists, engineers, and educators from both industry and top academic institutions, who are excited to offer you and your team our expertise through basic and more advanced courses. We can also perform case studies and provide technical support services.
Basics of Bioprocessing
Bioprocess Overview
Bioprocess Overview course is designed for the individual that is new or has limited bioprocessing experience. The class discusses a very typical generic batch antibody manufacturing process starting with the master cell bank, follows at a high level each of the intermediate unit operations through cell culture expansion into separations/chromatography, and finally concluding with bulk drug substance fill. The learning objective is to provide a big picture view of bioprocessing and some basic knowledge of the unit operations employed in the manufacture of antibody therapies.
Pathogen Safety
The Pathogen Safety Course provides a conceptual overview for the development of a safe and compliant process for biomanufacturing. The lecture will cover general principles of pathogen safety for building robust manufacturing processes. Some specific highlights are keys to mitigating risk, materials sourcing, process testing, and reduction strategies for implementation of biological processes into commercial operations.
Principle Driven and Conceptual Learning in Bioprocessing
Principles of Cell Culture
Principles of Cell Culture course is designed for relatively new cell culture operators and it provides a conceptual overview of the core principles driving cell culture.Emphasis is placed on biological and technological factors affecting product quality, and general manufacturing processes. At the end of this course, the attendee will reach a clear understanding of how cloning, aseptic techniques, growth conditions, and other logistics factors affect manufacturing processes.

Principles of Tangential Flow Filtration
Principles of Tangential Flow Filtration (TFF) includes a conceptual description of crossflow filtration and a presentation on TFF applied to biomanufacturing and biopharma cases.The theory will cover the core concepts in cake layer filtration, efficiency analysis, and fouling. The applied part of this lecture will describe the different types of TFF modalities, with practical tips about operation and process optimization.

Principles of Chromatography
Principles of Chromatography course is designed for the relatively new bioprocess operator/development researcher looking to increase knowledge in standard chromatography chemistries and column packing principles. The class focuses on chromatography foundations including standard bioprocess chemistries like Protein A, hydrophobic interaction, and ion exchange. The learning objective is to understand the basics of column packing and understand separations based on size exclusion, charges, hydrophobicity and Fc/Protein A interactions.
Technical and Product Related Learning
Planova™ Operators Course
The Planova™ Operators course includes an in-depth lecture on the theory of nanofiltration and a virtual lab demonstration. The classroom style lecture and the lab demonstration are centered around virus removal methods and are conducted by live speakers in real time.The demonstrations will key in on the robust functionality of Planova™ filters, and on their simple operation procedure.

Microfiltration Operators Course
This BioOptimal/Microza Microfiltration Operators course includes a detailed lecture on the use of hollow fiber filters in microfiltration to obtain superior throughput at challenging cell densities. The classroom style lecture highlights perfusion applications. Our lab demonstration focuses on a recirculation loop using a hollow fiber filter connected to a glass bioreactor, and we will use a low shear pump for flow control. This training is conducted by live speakers in real time.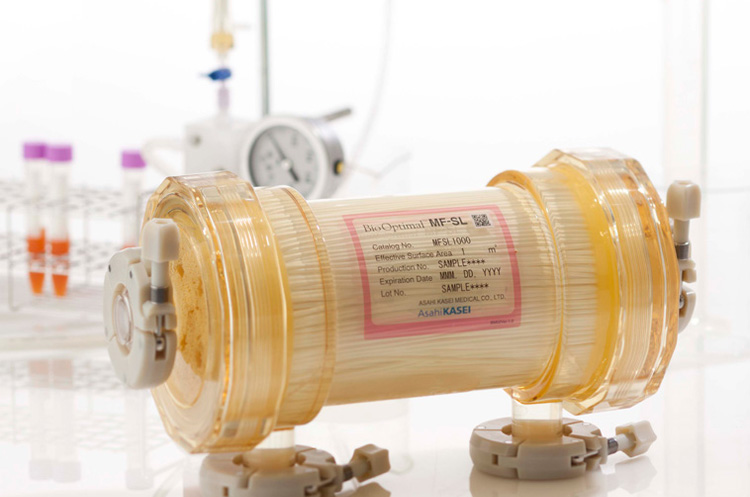
Planova™ Virus Filtration Integrity Testing
The Planova™ Virus Filtration Integrity Testing for Manufacturing course is a detailed presentation concerning the integrity tests employed when using Planova™ virus filters.These tests include the VLT (Visual Leak Test) and the GPT (Gold Particle Test). The lecture will describe how the mechanisms associated with each of the tests and discuss why these need to be performed. Additionally, there will be a troubleshooting section designed to prevent common mistakes made during performance of the tests. Finally, there will be a presentation of a large-scale execution of the tests as would be done in manufacturing.
This class is for those individuals currently or potentially in the future going to perform the VLT and GPT at large scale in manufacturing as well as for those individuals developing virus filtration processes for manufacturing operations.

Additional Learning Resources
Biopulse: the Industry Experts Webinar Series
Future of Bioreactor Technology and Data Trending
This panel discussion aims to shed light on the future of bioreactor technologies with gathered insights from industry and academic thought leaders. Experts in process automation, real-time process analytical technology, modeling, and cloud-based data management will explore the direction of cell culturing in biopharmaceutical manufacturing.

