About Us About Asahi Kasei Bioprocess
Our Business Overview
Pioneering Biologics Safety and Manufacturing Efficiency
Asahi Kasei Bioprocess is a global solution provider to biologics manufacturing industry. We aim to help biologics manufacturers safely and efficiently produce medicines that patients can trust, by dependably supplying innovative yet exceptionally reliable bioprocess consumables, equipment and scientific support services.While Asahi Kasei Bioprocess has established a market leadership in virus filtration, we aim to be the global leader and experts in contributing to biologics safety and manufacturing efficiency by leveraging our core technology, brand and services in virus removal.
As a part of the Asahi Kasei Group's sharpening focus on healthcare-related fields, Asahi Kasei Bioprocess is steadily developing its range of advanced manufacturing solutions for the bioprocess industry.
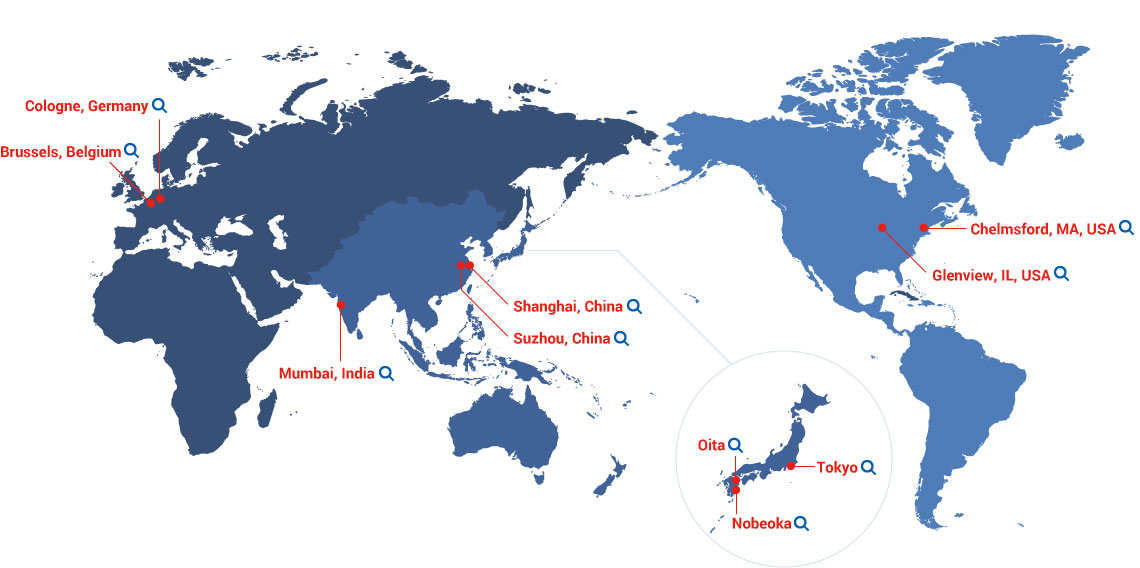
Our Global Network
Our History
In 1980s, rise of awareness on the need to further strengthen viral safety of plasma-derived pharmaceutical products emerged globally due to a number of contamination cases that impacted thousands of patients, specifically those suffered from haemophilia.
Recognizing the issue, Asahi Kasei rose to develop filters specifically designed to remove viruses from plasma-derived pharmaceutical products, leveraging its solid expertise in fibers and filtration technology. The filters were later named "Planova™", originated from the words "Plasma" and "Nova" (which means "New Star" in Latin). The world's first virus filter was Planova™ 35N, with membrane mean pore size of 35 nm, and through collaboration with various pharmaceutical companies, universities, research institutions and regulatory agencies, was successfully evaluated to be able to remove viruses such as HIV and HBV. It was the start of virus filtration technology that later become industry standard.
From there, our road was not easy, and our path to being the leader and experts in the industry does not happen overnight. There was time when we were faced with challenges to continue our business, but with commitment from our staff and support from the industry, we will continue to strive to fulfill our mission: Pioneering Biologics Safety and Manufacturing Efficiency.
While we have accumulated over 30 years of trusted use in the production of biotherapeutics with Planova™ virus removal filters, we continue to innovate solutions, products and services to help our customers in achieving biologics safety and manufacturing efficiency.
Recognizing the issue, Asahi Kasei rose to develop filters specifically designed to remove viruses from plasma-derived pharmaceutical products, leveraging its solid expertise in fibers and filtration technology. The filters were later named "Planova™", originated from the words "Plasma" and "Nova" (which means "New Star" in Latin). The world's first virus filter was Planova™ 35N, with membrane mean pore size of 35 nm, and through collaboration with various pharmaceutical companies, universities, research institutions and regulatory agencies, was successfully evaluated to be able to remove viruses such as HIV and HBV. It was the start of virus filtration technology that later become industry standard.
From there, our road was not easy, and our path to being the leader and experts in the industry does not happen overnight. There was time when we were faced with challenges to continue our business, but with commitment from our staff and support from the industry, we will continue to strive to fulfill our mission: Pioneering Biologics Safety and Manufacturing Efficiency.
While we have accumulated over 30 years of trusted use in the production of biotherapeutics with Planova™ virus removal filters, we continue to innovate solutions, products and services to help our customers in achieving biologics safety and manufacturing efficiency.
| 1987 | Establishment of "Bemberg Microporous Membrane" Project, which later became Planova™ Division and developed Planova™ filters |
|---|---|
| 1989 |
Launch of Planova™ 35N virus removal filters as the world's first filters specifically designed for virus removal in biotherapeutics manufacturing
 |
| 1993 | Launch of Planova™ 15N virus removal filters to capture smaller viruses |
| 1994 | Foundation of TechniKrom, Inc. in Illinois, USA (which later became a part of Asahi Kasei Bioprocess) |
| 1997 | Official opening of Planova™ Plant in Nobeoka, Miyazaki, Japan |
| 1998 |
 |
| 2000 |
  |
| 2001 |
Launch of Planova™ 20N virus removal filters that capture small viruses while accommodating needs for higher flux
 |
| 2003 |
|
| 2004 | Opening of a new regenerated cellulose hollow fiber spinning facility for Planova™ filters in Nobeoka, Miyazaki, Japan (current Planova™ Spinning Fab 1) |
| 2005 |
|
| 2006 |
Launch of IBD inline buffer dilution system by TechniKrom, Inc. (which later became a part of Asahi Kasei Bioprocess)
 |
| 2009 |
 |
| 2010 |
|
| 2011 |
  |
| 2012 |
|
| 2013 |
|
| 2018 |
|
| 2019 |
 |
| 2021 |
|
