Products & Services Audit Reports
- HOME
- Products & Services
- Audit Reports
Audit Reports Availability
Audit reports for Planova™ Plant and Planova™ Oita Plant are now available for purchase from Rx-360, an international pharmaceutical supply chain consortium.
Purchasing these audit reports allows biopharmaceutical companies to obtain audit reports for Planova™ manufacturing facilities without having to conduct the audits directly, which could potentially help to save time and costs. Other features and benefits of purchasing the audit reports are as follows:
Purchasing these audit reports allows biopharmaceutical companies to obtain audit reports for Planova™ manufacturing facilities without having to conduct the audits directly, which could potentially help to save time and costs. Other features and benefits of purchasing the audit reports are as follows:
- Companies could save time and cost of traveling to Miyazaki and Oita, Japan.
- Companies could save cost and labor of contracting third-party auditors to conduct in Japan.
- Both FDA and EMA accept audits conducted by third-party auditors, including these reports.
- Reports can be purchased by Rx-360 members or non-members.
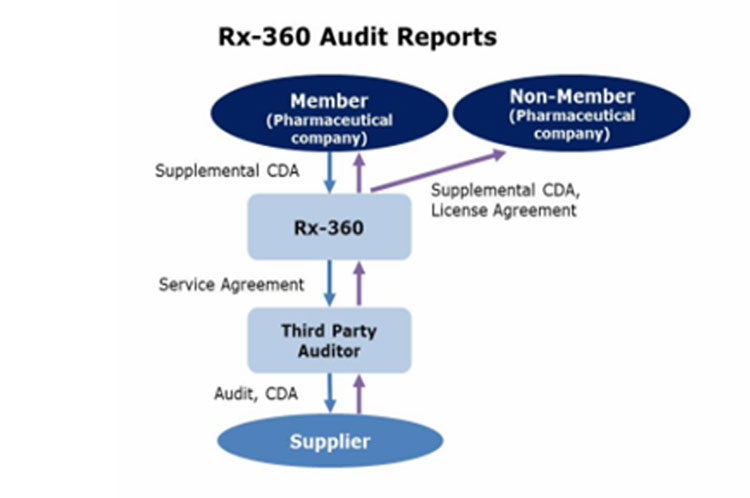
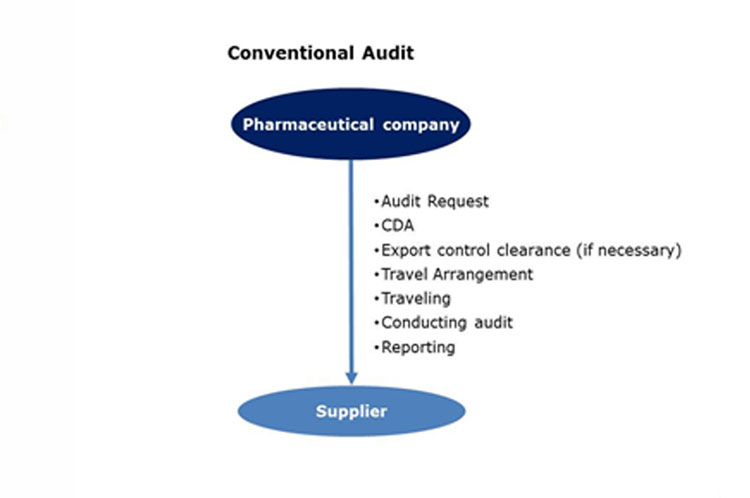
If you are interested in licensing the reports, please contact “Brian Shipley” at bshipley@rx-360.org.
Please indicate the following audit number in your inquiry: Planova Plant (JA-2723) , Planova Oita Plant (JA-2724)
Please indicate the following audit number in your inquiry: Planova Plant (JA-2723) , Planova Oita Plant (JA-2724)
| Audit Number | Site | Start Date | End Date | Audit Method | Audit Guideline Used |
|---|---|---|---|---|---|
| JA-2723 | Asahi Kasei Medical Co., Ltd. - Nobeoka, Japan | 12/13/22 | 12/14/22 | Onsite | Basic Chemicals / Raw Materials; Supply Chain Security |
| JA-2724 | Asahi Kasei Medical Co., Ltd. - Oita-city, Japan | 12/15/22 | 12/15/22 | Onsite | Basic Chemicals / Raw Materials; Supply Chain Security |
| Planova™ Plant | Planova™ Oita Plant | |
|---|---|---|
| Planova™ 15N, 20N & 35N and 75N | ✓ | ✓ |
| Planova™ BioEX | ✓ | ✓ |
| Asahi Integrity Test Solution Kit | ✓ |
Please note that customers purchasing Planova™ or Planova™ BioEX may receive filters manufactured either in Planova™ Plant or Planova™ Oita Plant, and thus may need to purchase audit reports from both plants.
Should you have any questions regarding these audit reports, please contact us.
- More information purchasing audit reports from Rx-360 can be found in the following page:
http://rx-360.org/audit-programs/purchase-audit-reports/
Should you have any questions regarding these audit reports, please contact us.
