Products & Services Details for Planova™ S20N
- HOME
- Products & Services
- Planova™ S20N Virus Removal Filters
- Details for Planova™ S20N

For removing viruses in biologicals,
from antibody-based therapeutics to plasma-derived products
Key Features
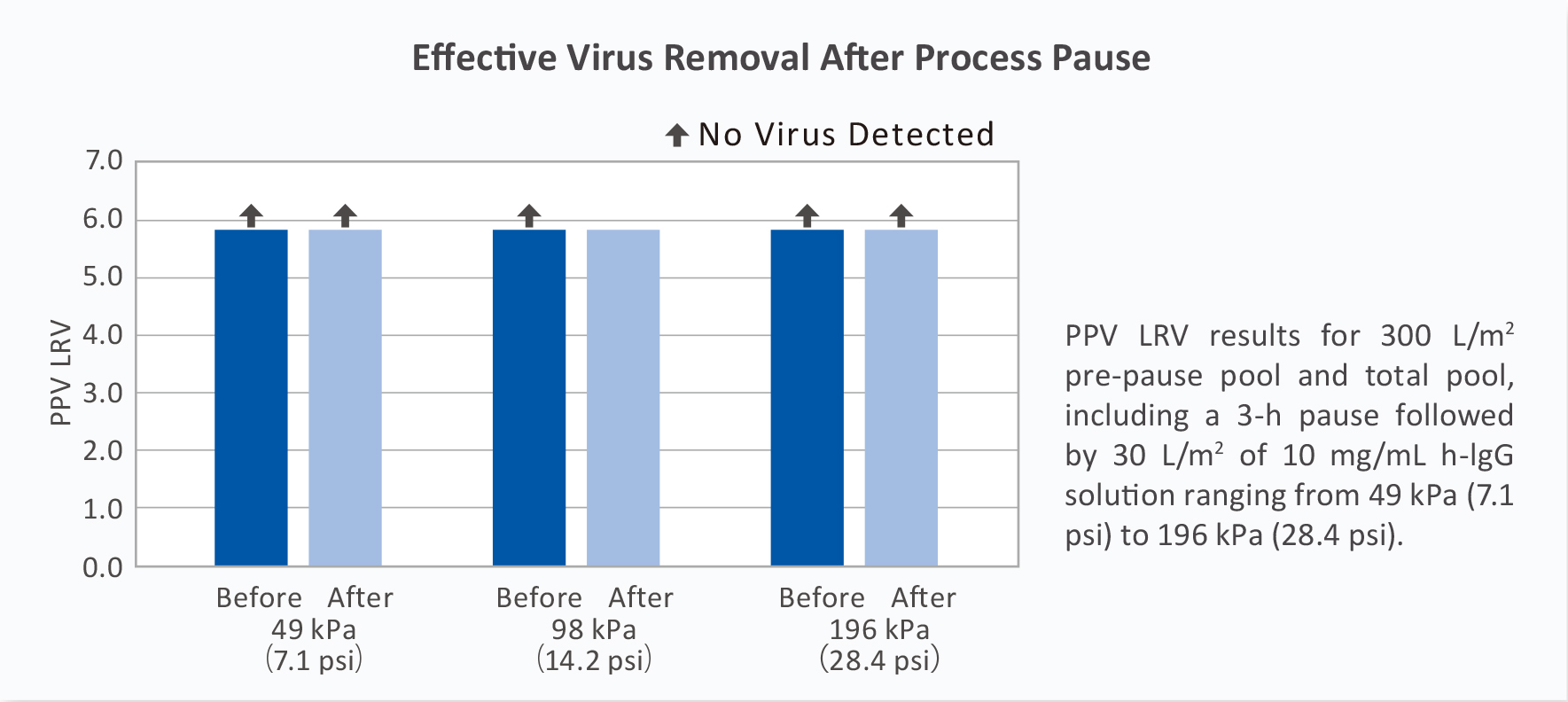
■ Robust Virus Removal Capability
Planova S20N shows high virus LRV with various viruses and process designs
Planova S20N shows high virus LRV with various viruses and process designs
■Processing More, Faster
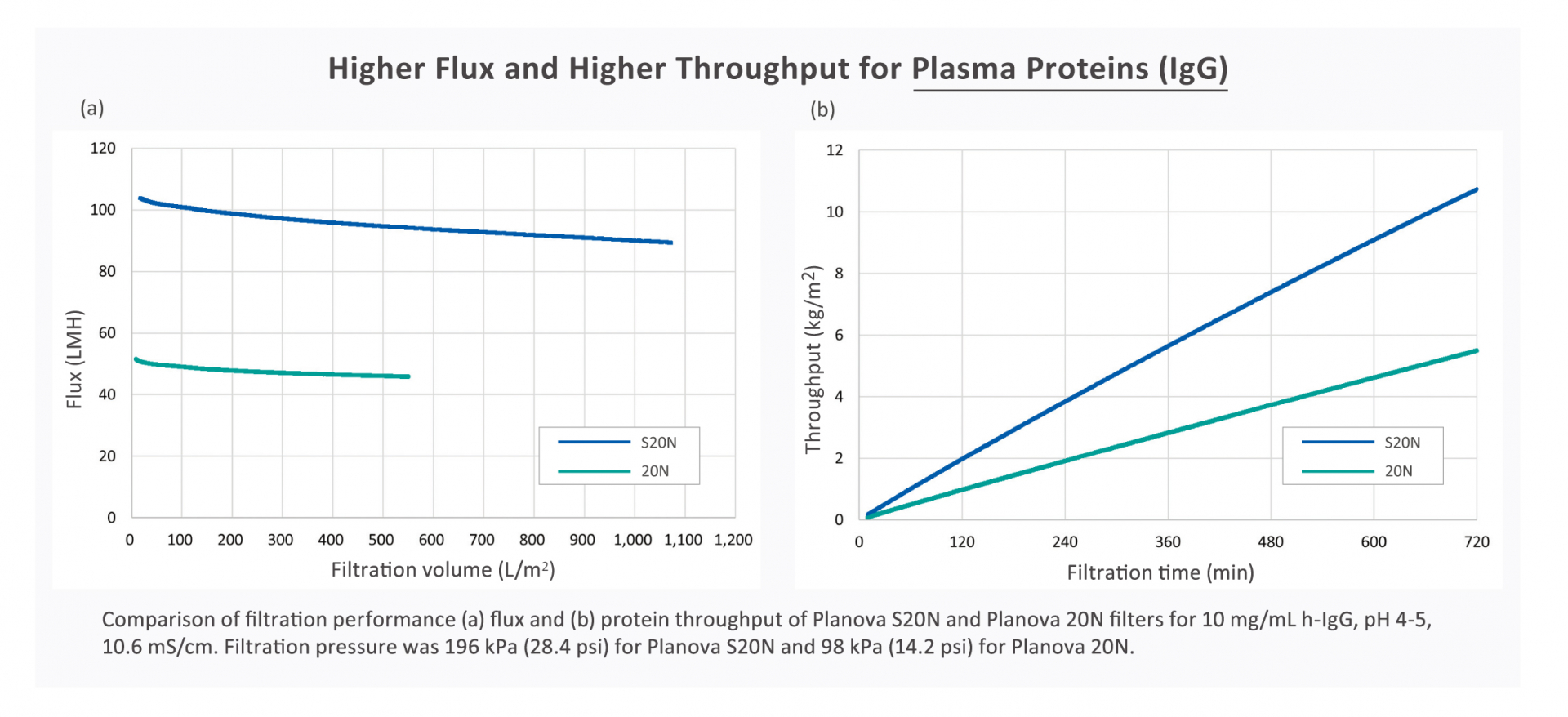
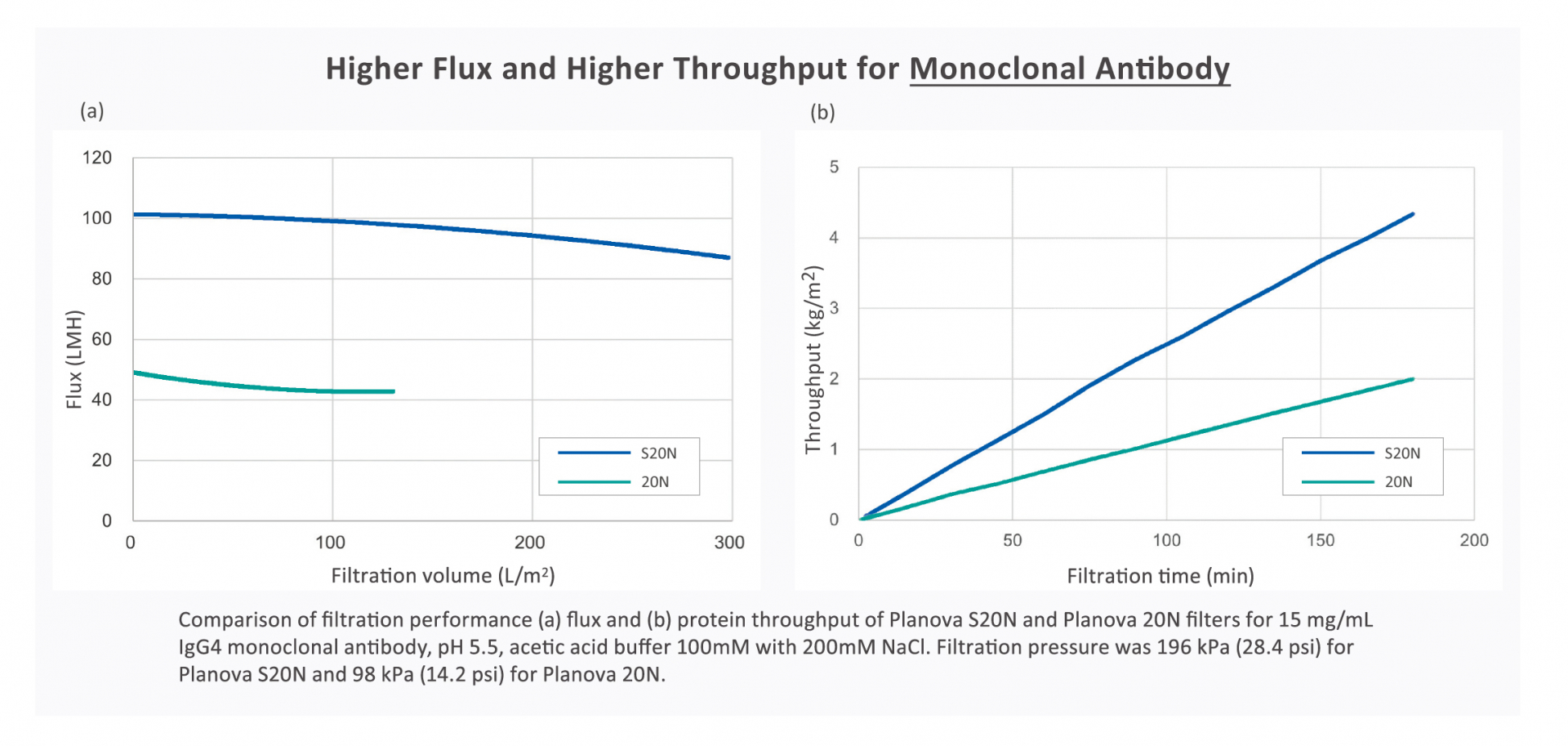
Superior Regenerated Cellulose Withstands Higher Pressure
The superior regenerated cellulose membrane of Planova S20N withstands higher filtration
pressure and resists clogging to deliver stable filterability with higher flux and throughput.
Superior Regenerated Cellulose Withstands Higher Pressure
The superior regenerated cellulose membrane of Planova S20N withstands higher filtration
pressure and resists clogging to deliver stable filterability with higher flux and throughput.
■Improved Operational Efficiency
»» Just like other Planova filters, Planova S20N arrives at your site “ready to use” ─ no pre-wetting or sterilization is required prior to use in filtration
»» The post-use integrity test is simplified to only a leakage test – no GPT* required
»» Planova S20N will contribute to operational efficiency during process scale-up and manufacturing
»» Just like other Planova filters, Planova S20N arrives at your site “ready to use” ─ no pre-wetting or sterilization is required prior to use in filtration
»» The post-use integrity test is simplified to only a leakage test – no GPT* required
»» Planova S20N will contribute to operational efficiency during process scale-up and manufacturing
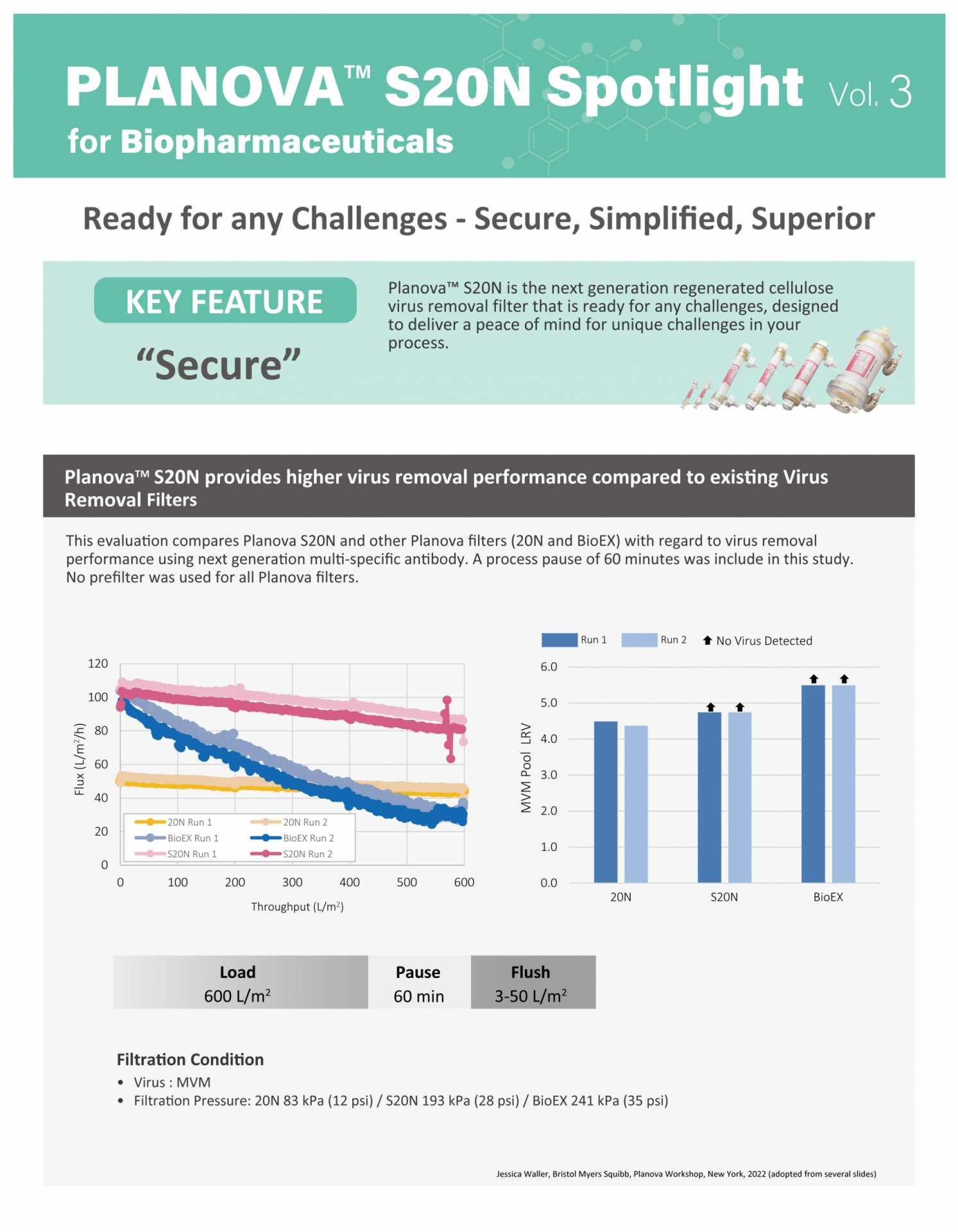
Planova S20N Spotlight
Flyer with data focusing on each of the Planova S20N features