Products & Services Planova™ BioEX Virus Removal Filters
- HOME
- Products & Services
- Planova™ BioEX Virus Removal Filters
Your First Choice Virus Filtration Partner that You Can Absolutely Rely On
Planova™ BioEX's superior filtration performance, combined with its robust virus removability and unrivalled quality, makes it an excellent virus filter choice for your biopharmaceutical products. What is more, we do not just offer the technology, but we support it with science and wealth of experience: our virus filtration experts are always passionate to help you with your process. Explore more how we can work together to maximize the potential of our technology used with your technology.Planova BioEX Spotlight Vol1.pdf[PDF:2.8MB]
Planova BioEX Spotlight Vol.2.pdf[PDF:2.7MB]
Planova BioEX Spotlight Vol.3.pdf[PDF:1.2MB]
Featured Supporting Documents
You need to login to download the files.
Quick links to selected Data Sheets, Product Manuals or Validation Reports related to the product.
Featured Articles
Quick links to selected literature, case studies or articles related to the product.
- Validation and implementation of Planova™ BioEX virus filters in the manufacture of a new liquid intravenous immunoglobulin in China", Ma, S. et al.,
- Development of small-scale models to understand the impact of continuous downstream bioprocessing on integrated virus filtration, Scott Lute, Julie Kozaili, et al., Biotechnol. Prog. 2020, e2962
- Clarification Impurities Impact Viral Filtration of a Therapeutic Antibody_Ryan Zolyomi_2018_The 21st Planova™ Workshop
- Meeting Planova and Selecting BioEX for Our Monoclonal Antibody Manufacturing Process_Yumiko Masuda_2018_The 21st Planova™ Workshop
Application
The Go-To Virus Filter for Various Target Molecules
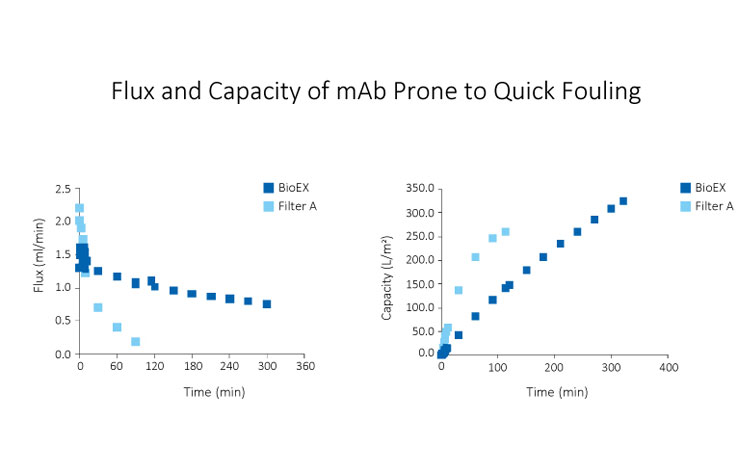
Planova BioEX filters work excellently with various biopharmaceuticals, even with those that are prone to quick fouling. Examples of proteins in which our customers experienced difficulty in filtration include monoclonal antibodies, fusion proteins, bispecific antibodies, antibody-drug conjugates, labile enzymes, recombinant coagulation factors, as well as plasma-derived immunoglobulins. It is the next generation virus filter that you can rely to work with your protein molecules.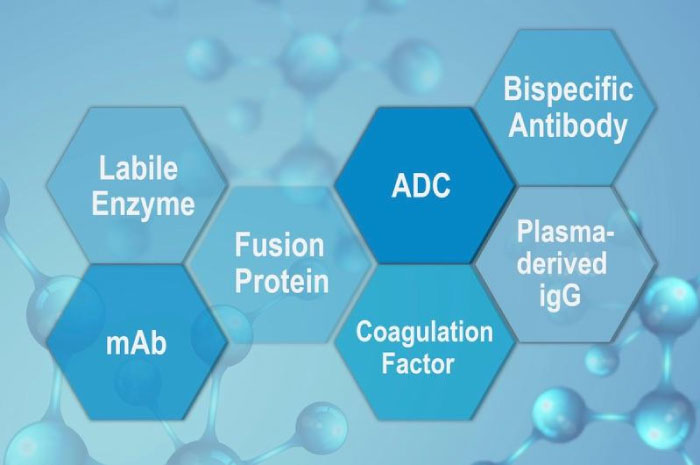
Features & Performances
The Filter that You Can Rely to Work with Your Molecules
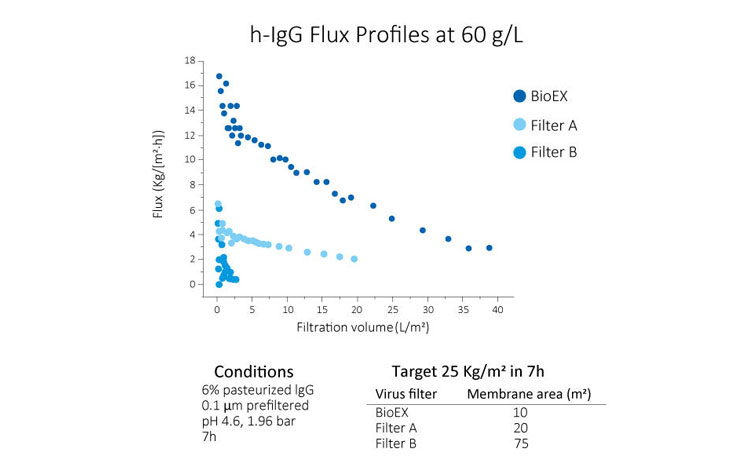
Various evaluations using Planova BioEX, such as those shown in the graphs below, have shown that the filter demonstrate excellent filterability with various molecules considered difficult to filter. This property of Planova BioEX makes it a powerful tool for virus filtration in process development phase of novel proteins.Fast yet Stable Performance for Efficient Filtration within the Allowable Time Frame
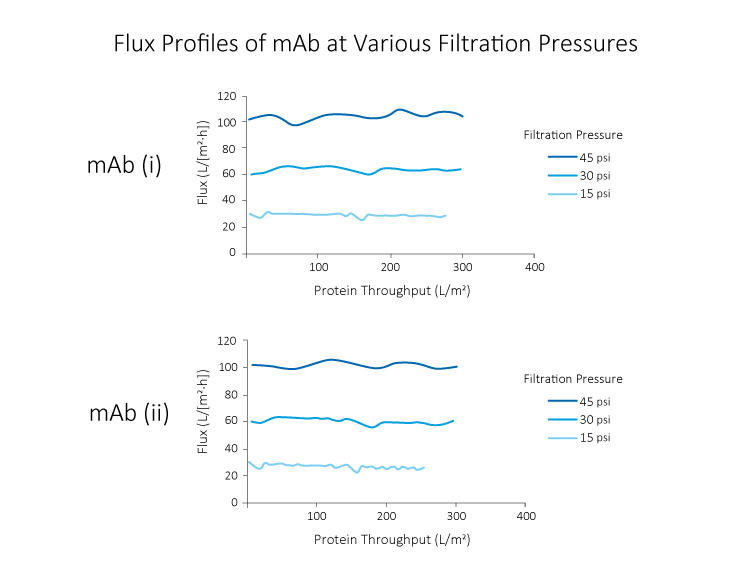
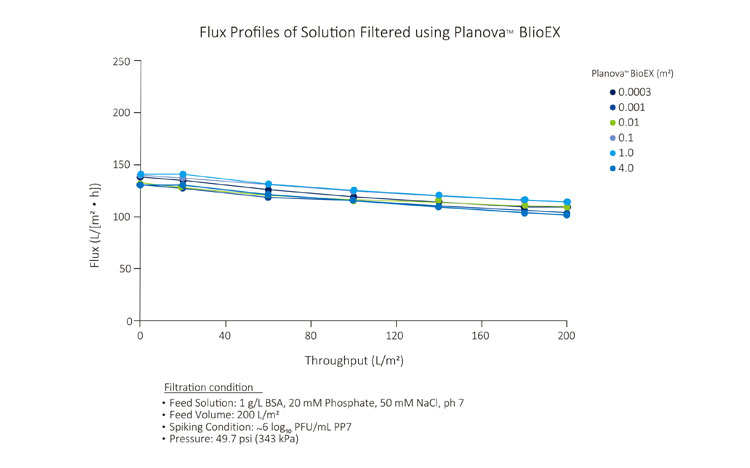
High pressure tolerance of Planova BioEX, contributed by its unique polyvinylidene difluoride (PVDF) membrane, gives the filter a high flux filterability. This allows for completion of a virus filtration process within the allowable time frame, typically four to eight hours within one manufacturing shift.Furthermore, Planova BioEX's performances are shown to be consistent with minimal flux decay throughout filtration runs under various conditions such as high pressure, high concentrations or large volume filtration. This stable performance of Planova BioEX makes it an efficient and reliable virus filter for each filtration run. For large volume filtration, the high capacity feature of Planova BioEX will allow for less filter surface area needed.
Robust Virus Removal Capability for Confidence in Virus Safety
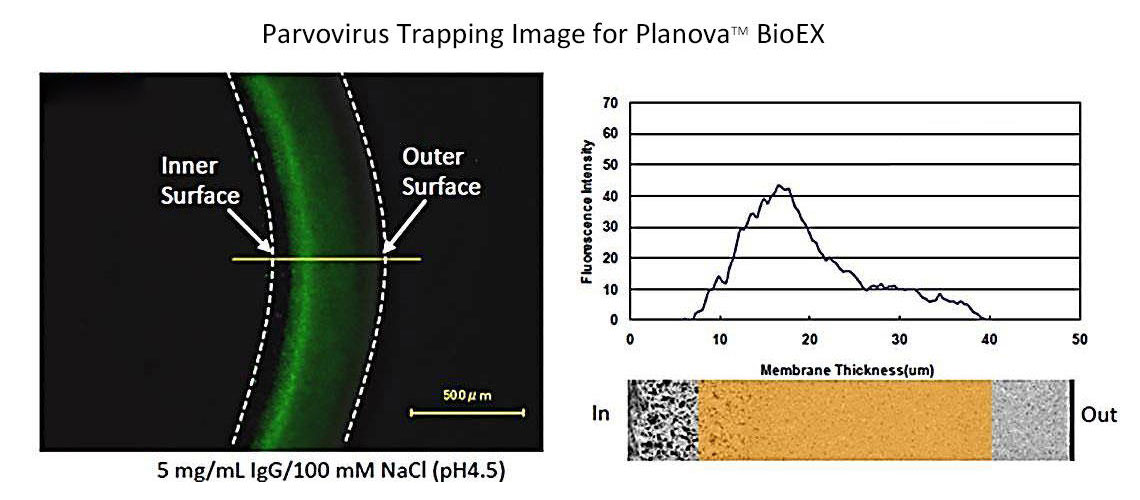
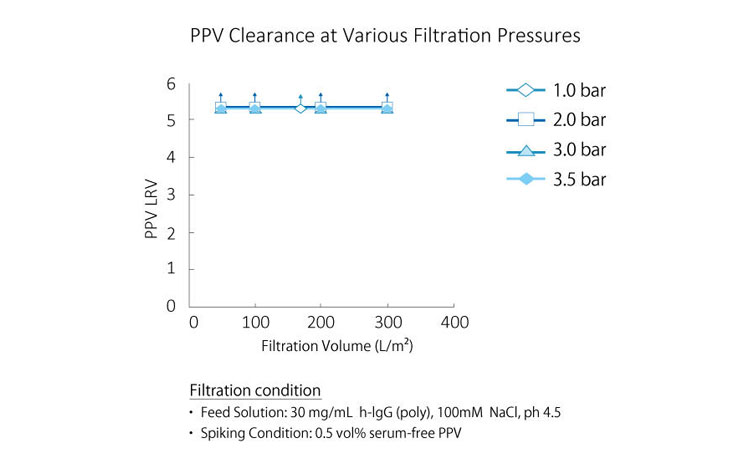
Virus filters are designed to remove viruses from filtered solution, and robust virus filters show high virus removability even under changing filtration conditions. Planova™ BioEX's thick membrane structure allows trapping of viruses over a large membrane region, making it an efficient virus removal filter.Furthermore, Planova™ BioEX's robustness allows for consistent virus removability with various molecules, under various conditions, such as different buffer conditions and different filtration pressures. This feature contributes to confidence in viral safety of customers' filtered products.
| pH | Conductivity (mS/cm) |
MVM Pool LRV | ||
|---|---|---|---|---|
| Mab 2 | Mab 1 | Mab 3 | ||
| pI 7.2 | pI 8.6 | pI 9.0 | ||
| 4 | 20 | ≥5.02 | ≥5.52 | 5.45 |
| 8 | 20 | ≥5.53 | ≥5.53 | ≥5.62 |
| 4 | 3 | ≥5.48 | ≥5.07 | ≥5.02 |
| 8 | 3 | ≥5.33 | ≥5.40 | ≥4.87 |
| 5.5 | 7 | ≥5.07 | ≥4.87 | ≥5.20 |
Joshua Goldstein, Janssen, 2014 Planova Workshop (adapted)
Consistent Quality for Smooth Scaling Up
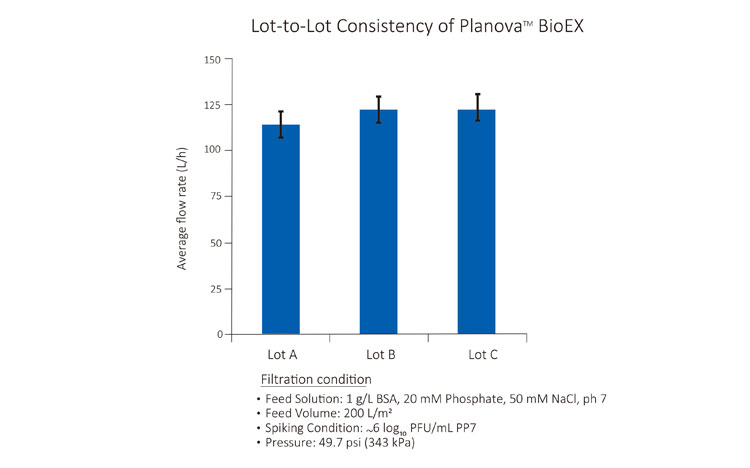
It is necessary for virus filters to have consistent performance from developmental to manufacturing scales, so as to improve the accuracy of scale-up calculation during process development phase. It is also crucial for virus filters to demonstrate consistent performance throughout different lots, so as to prevent unnecessary 'surprises' during development or manufacturing. Designed with hollow fiber membranes and manufactured under a trusworthy Quality Management System, Planova BioEX demonstrate consistent performance regardless of effective surafce area sizes or manuafcturing lots. Customers can have a worry-free scale-up process with our reliable virus filters.Easy Operation with Full Support from the Experts
Having unique hollow fiber membranes and filter housing design does not make Planova BioEX a difficult filter to install and operate. The filter's intact module does not require additional stainless steel housing, which minimizes installation error during setup and thereby ensuring virus filtration system integrity.
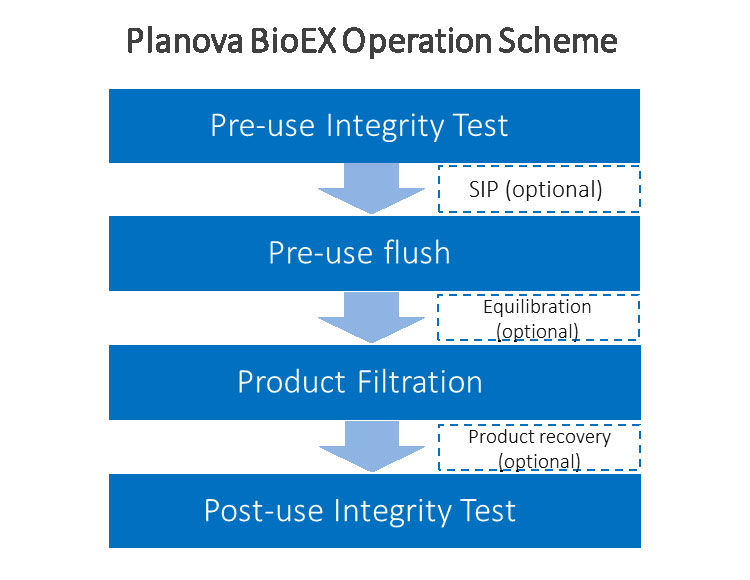
Operating virus filtration with Planova BioEX is simple and worry-free. Each filter is released in wet and sterilized conditions, thereby improving the ease of set-up and preparation that need to be done during operation. Users only need to perform integrity test and flushing (with options to perform in-line SIP and equilibration) prior to product filtration.
Not only we provide supporting documentation which can be accessed via the Technical Documents, our technical support team will provide full support with regards to process design, installation, operation and integrity testing of our products. Various trainig courses are also available to further familiarize users with technical aspects and operation of our filters and their related products.
Integrity testing of Planova BioEX is easy with an air-diffusion-based Visual Leakage Test, which is conducted by bubbling obeservation. If automation is required, an automated Planova Leak Tester can also be used. The simplicity of the process allows virus filtration with Planova BioEX and its integrity test to be completed within one manufacturing shift for most production cases. Please visit the Integrity Test Section of this page for more information about Planova BioEX Integrity Test.
Not only we provide supporting documentation which can be accessed via the Technical Documents, our technical support team will provide full support with regards to process design, installation, operation and integrity testing of our products. Various trainig courses are also available to further familiarize users with technical aspects and operation of our filters and their related products.
Integrity testing of Planova BioEX is easy with an air-diffusion-based Visual Leakage Test, which is conducted by bubbling obeservation. If automation is required, an automated Planova Leak Tester can also be used. The simplicity of the process allows virus filtration with Planova BioEX and its integrity test to be completed within one manufacturing shift for most production cases. Please visit the Integrity Test Section of this page for more information about Planova BioEX Integrity Test.

Specifications & Parameters
Material Specifications
| Planova BioEX Filter Material Specifications | ||||||||
|---|---|---|---|---|---|---|---|---|
| Effective surface area (m²) | 4.0 | 1.0 | 0.1 | 0.01 | 0.001 | 0.0003 | ||
| Component | Hollow fiber membrane | Hydrophilic modified polyvinylidene fluoride | ||||||
| Housing and headers | Polycarbonate | |||||||
| Sealant | Polyurethane | |||||||
| O-rings | Silicone | |||||||
| Nozzle plugs | Silicone | - 1 | ||||||
| Nozzle caps | - | Silicone | ||||||
| Ferrule caps | Polycarbonate | - | ||||||
| Luer lock plugs | - | Polycarbonate | ||||||
| Balloon cap holders | Polycarbonate | - | ||||||
| Gaskets 2 | Silicone | - | ||||||
| Balloon caps | Silicone | - | ||||||
| Nozzle stoppers | - | Silicone | - | |||||
| Clamp bands | Polysulfone | - | ||||||
| Threaded Clamps | Clamp bolts | - | Polypropylene | - | ||||
| Clamp nuts | - | Polypropylene | - | |||||
| Locknuts | Polycarbonate | - | ||||||
| Supplied as | Filled with purifled water 3 | |||||||
| Sterilization method | Autoclaving | |||||||
| Packaging format | Packed individually in sterilization bags | |||||||
1 Not applicable
2 Gaskets are used for connecting quick clamps to nozzles of Planova BioEX filters with effective surface areas of 4.0 m2, 1.0 m2 and 0.1 m2.
3 Purified water in 4.0 m2, 1.0 m2 and 0.1 m2 Planova BioEX filters contains <0.1% NaCl.
2 Gaskets are used for connecting quick clamps to nozzles of Planova BioEX filters with effective surface areas of 4.0 m2, 1.0 m2 and 0.1 m2.
3 Purified water in 4.0 m2, 1.0 m2 and 0.1 m2 Planova BioEX filters contains <0.1% NaCl.
Operating Parameters
| Operating pressure (transmembrane pressure) |
≤ 343 kPa |
|---|---|
| Operating pH | 2-9 |
Integrity Test
Integrity test of Planova BioEX is conducted to ensure the integrity of the filter when used in filtration process. The following qualities allow for a simple user integrity test required to be conducted at the user end: The membrane used in Planova BioEX filters is made of hydrophilic modified polyvinylidene fluoride (PVDF), which provides greater stability and material strength. Furthermore, various quality control tests during the production ensure that each hollow fiber membrane of Planova BioEX filters are free from defects, specifically those caused by improper pore size distribution or gross defects in the form of broken fibers or pinholes, which may affect virus removability of the filters.
Users are to conduct integrity test for Planova BioEX twice: once before filtration to detect gross defects that may occur during shipment, and another after filtration, to detect defects that may occur during filtration.
The integrity test for Planova BioEX is easy with an air-diffusion-based test, which can be performed either manually via visual detection of pressurized air bubbling through the hollow fibers using the Visual Leakage Test method, or automatically via measurement of airflow through the hollow fibers using the Planova Leak Tester (for filters with effective surface area size of 0.1 m2 or larger).
The integrity test for Planova BioEX is easy with an air-diffusion-based test, which can be performed either manually via visual detection of pressurized air bubbling through the hollow fibers using the Visual Leakage Test method, or automatically via measurement of airflow through the hollow fibers using the Planova Leak Tester (for filters with effective surface area size of 0.1 m2 or larger).
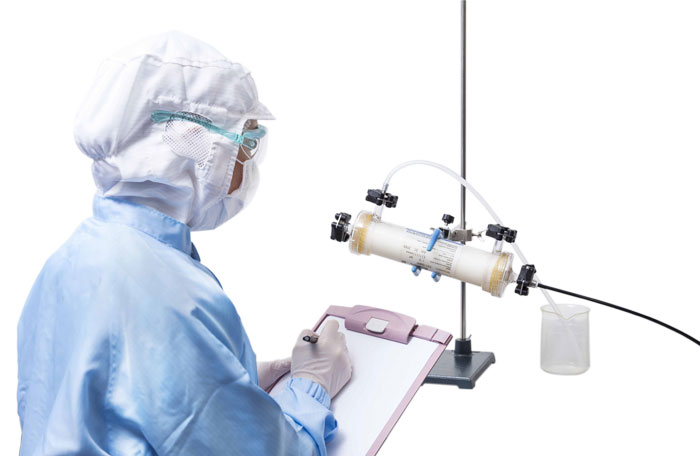
Visual Leakage Test

Leakage Test with Planova Leak Tester
The table below lists the catalog number for Planova™ Leak Tester and its parts. Please contact us and cite the number below to order.
*Not available in selected country/regions.
| Planova Leak Tester | |
|---|---|
| Product | Catalog number |
| Controller (with interface for US) | PLT-AM10MU |
| Controller (with interface for Europe) | PLT-AM10ME |
| Pneumatic Circuit Unit | PLT-AM10K0 |
| Tray Set | PLT-AM10TS |
To learn more about WEEE compliance in Europe for the Planova Leak Tester, please follow the links below.
Ordering information
The table below lists the catalog number for Planova BioEX virus removal filters. Please contact us and cite the number below to order.
| Planova BioEX Filters | ||
|---|---|---|
| Filter effective surface area size (m²) | Catalog number | |
| Planova BioEX | 4.0 | EX4-0000 |
| 1.0 | EX1-0000 | |
| 0.1 | EXZ-1000 | |
| 0.01 | EXZ-0100 | |
| 0.001 | EXZ-0010 | |
| 0.0003 | EXZ-0003 | |